fara Australia Patient & Family Information Forum 2024, part 1 of 5
Once every few years, fara gathers the leading FA researchers in the country and brings them to Brisbane so we can hear from them first-hand, where their research is up to and where they hope it’ll go next.
We’re blessed to have working here in Australia, some of the leading FA researchers in the world. These folks do the initial research, identifying how FA works or how bodies impacted by FA don’t, and we tend to leave the drug development to the US where there is more access to the huge investment needed for drug development.
fara organised such a forum at UQCCR (where the Brisbane FA Clinics are held) on Feb 29th, 2024. I was lucky enough to be able to attend those presentations, but having rewatched them just now, I realise that I missed much that was presented at the time. Fortunately fara organised for them all to be recorded and you can view them in your own time at https://www.fara.org.au/news/watch-all-presentations-from-our-recent-patient-and-families-information-forum. Alternatively, you can read my summaries of what was presented (below and in a few more notes in coming weeks); or check out both and let me know if you think I’ve done a good job!
The session was opened by Brad Hyde, CEO of fara who noted the specialness of the date (29th Feb – Rare Disease Day, FA – very rare disease), also thanking Biogen who made the day possible. Biogen (one of the deep-pocketed drug companies I referred to above) who’ll present later in the day, own Skyclarys which on Rare Disease Day in 2023, was the first ever FA treatment approved by the FDA in America. Their presentation later will be about the road to getting TGA approval for Australia.
Before that though, we heard first up from Associate Professor Ian Harding. We’re delighted that Ian has recently moved from Monash in Melbourne to head up the Cerebellum & Neurodegeneration Research Group (CNRG) at QIMR Berghofer in Brisbane. If you can lie still for a few minutes and not get claustrophobic being inside a big plastic tube, Ian will make it worth your while as he and his team are finding that neuroimaging may be a more sensitive and useful tool, in identifying biomarkers, state/stage of FA and rate of progression than current methods (mFARS) in FA.
This is especially good news for wheelchair users as most trials don’t include us since mFARS doesn’t measure progression well once a person isn’t ambulant; and if we’re not in the trial then we’re not the expected primary beneficiaries of whatever treatment is being developed.
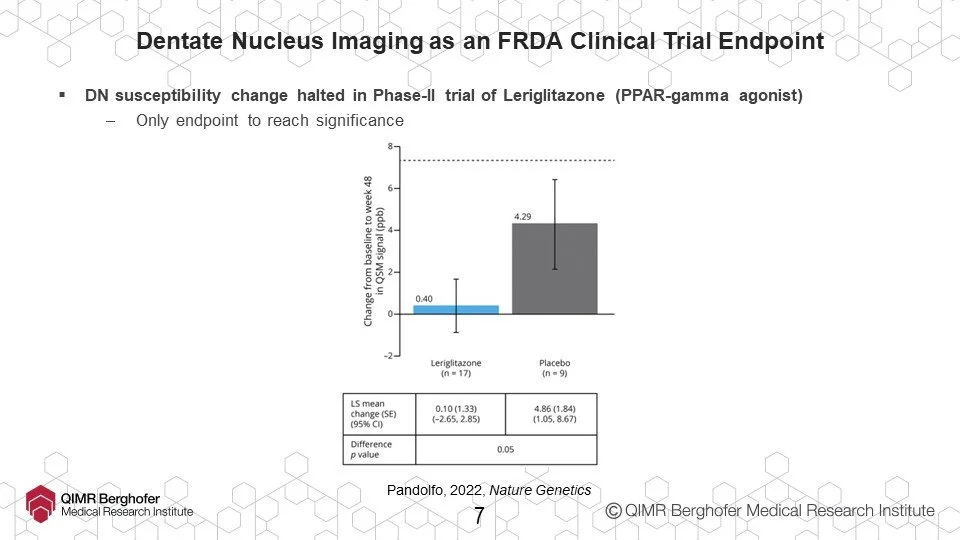
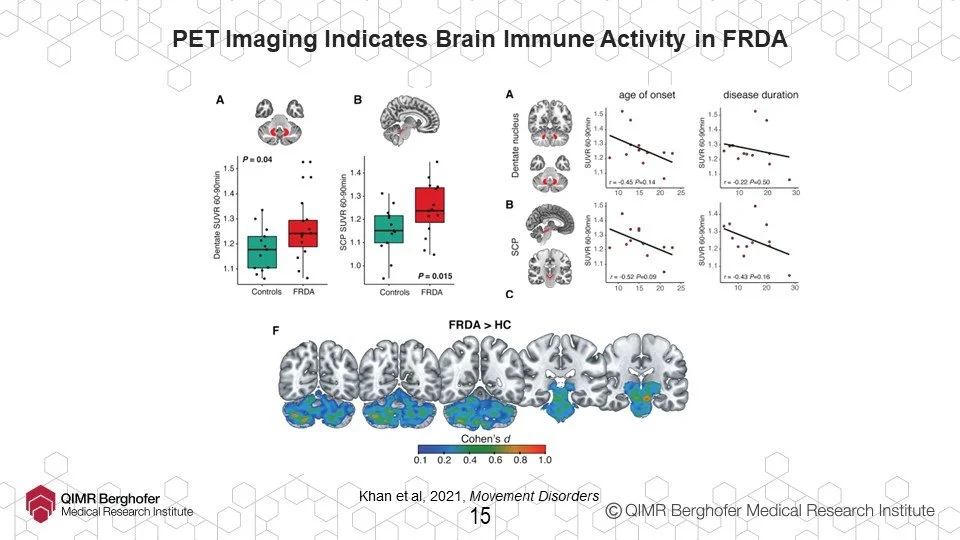
For example, dentate nucleus imaging. For the first time, in 2022, neuroimaging of dentate nucleus was used to demonstrate effectiveness of a drug in clinical trial.
In addition, the team’s work is showing that it should be possible to measure even more subtle changes or changes in more specific areas, and associate them with specific disease effects. This will indicate specific targets for treatment development and perhaps, crossover with development being done by teams addressing other diseases.
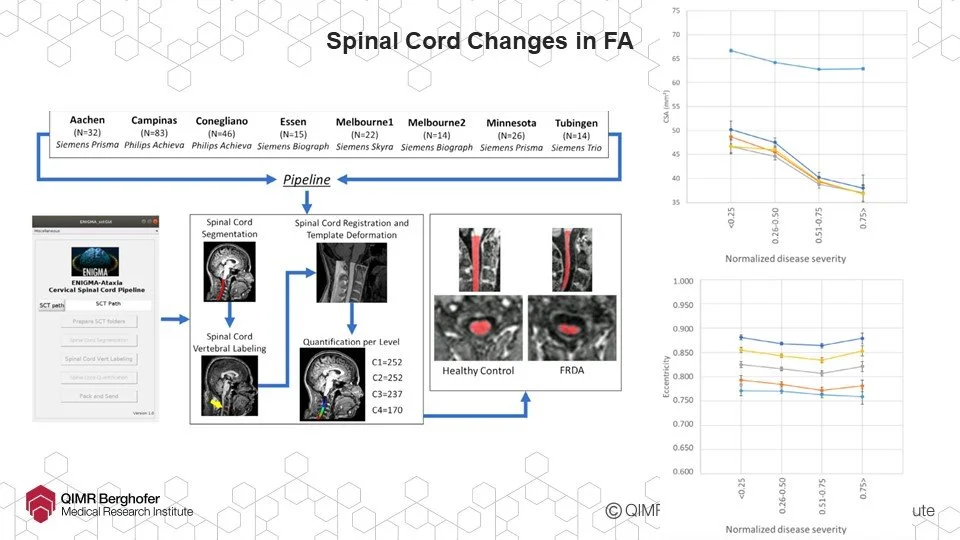
Speaking of identifying treatment target areas, working with researchers from the world-wide ENIGMA-Ataxia consortium, Ian’s team established that FA is associated with a ‘flattening’ of the spinal cord but that this ‘flattening’ reaches a limit and at a point appears to stop altogether. It’s likely this is degeneration of the sensory pathways but maintenance of motor instruction pathways which in turn should be the launch point for some promising new research work and treatment areas.
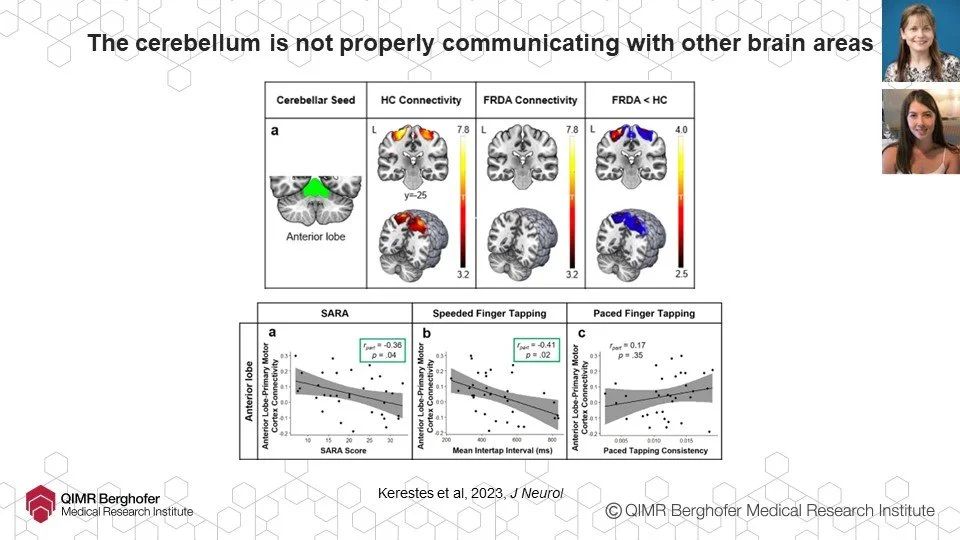
Many FAers know that the area of our brains most affected is the cerebellum (back of the head just above the neck), where motor instructions go from (or in our case, don’t). Ian’s team have been using imaging also to investigate how well signals are going from the cerebellum to other parts of the brain and have found that the quantity and efficiency of those signals is reduced in FA, suggesting that changes in brain networks, not just in the cerebellum, may contribute to FA progression.
Now Ian loves imaging of all kinds, so he used a different imaging process to review immune activity inside the brain in FA and other neurodegenerative diseases, again, specifically inside the dentate. Normally, this immune activity is good and when the body turns it on temporarily, it ‘flushes’ bad or dead cells out of the brain or body. Unfortunately, one of the things that happens when neurodegenerative diseases like FA are present is that the immune activity remains turned on permanently. This leads to a state called chronic neuroinflammation and isn’t good at all. D’you know how an adrenaline rush might enable someone to do a superhuman feat? Well, if the adrenaline kept flowing and that person tried to run a marathon, they might rush away at the start but their body would eventually break down entirely. It’s like that. It hasn’t been established yet whether this is a cause or effect of FA but now that it’s been identified conclusively, that’ll be the next step.
In Ian’s summary of the things above he reminded us that while imaging isn’t yet widely used outside of research, his work is showing how sensitive and therefore useful it can, and in future it’s hoped will, be.
Next up was Professor Mirella Dottori who leads a team at the University of Wollongong in NSW. Mirella started with an important note about where her kind of research fits into the broader picture of FA especially for those of us who live with it. Imagine a war (which isn’t difficult nowadays). Most immediately it’s essential to stop hostilities so people stop being hurt. That can equate with therapy – speech, physio, fitness, OT etc. Basically anything that improves quality of life.
It’s equally important also to understand what caused the war to break out in the first place since only by addressing the underlying cause can a new breakout be avoided in future. Equivalently in FA that’ll be the domain of gene therapies and drug treatments.
Unfortunately, it’s rarely straightforward to address the cause of anything (especially war) without unintended consequences in other areas, so that process can be complicated and arduous and takes a very long time. So in the meantime it’s important to address the mechanism – how problems arise – and to neutralise that. FA’s first and most significant problems are with balance. That’s a mechanism issue and that’s where Mirella’s research fits.
Her group works with Induced Pluripotent cells. Remember when you were just an egg? It’s unlikely you do since the few cells that made you you, weren’t yet brain cells so couldn’t form memories. Rather, they were pluripotent, with the power (“potent”) to become anything (“pluri”). Then those few cells divided, divided and divided again, all the way to becoming the you of today. Along the way, your cells became specialised so even as they divide nowadays, they’ll always stay specialised – skin, bone, heart cells etc. The cells that Mirella’s team use are skin cells from an FAer, but with very clever chemical engineering, the clock’s been turned back (the “induced” bit) so once again they’re “pluripotent” – they could be anything. Then, with more chemical engineering, these cells are guided to become cells of the nervous system.
Some reasons this is important as a starting point are that “cell lines” that research is done on are from an actual FAer and that until this process was developed nerve cells were very difficult to obtain. Also, the same cell lines can be shared among researchers, so Mirella’s group can be working on neurological issues while another team could be working on cardiac issues, but everyone can have the same confidence in one another’s research results.
Mirella’s work looks at proprioceptor sensory neurons. Within a muscle, there are proprioceptor neurons, which send signals to the brain about what the muscle “senses” about itself and motor neurons, which receive instructions from the brain about what the muscle should do (remember, Ian’s work suggested that the FAer’s spinal cord was losing sensory neurons but maintaining motor neurons which is why strength lasts longer for an FAer than control).
Before now, we had a general thinking that lack of frataxin meant that iron wasn’t processed efficiently within cells so there’d be sulphur-iron clusters which would cause mitochondrial stress and cell death. Mirella’s team have shown that even that description is too simplistic. There’s a Goldilocks Zone for mitochondrial stress, cells do need some to function but not too much. Using a number of different kinds of microscopic photography, they’re showing that shape also matters for mitochondria (long shapes are good and healthy, small and round not so much) and have established that there’s a Goldilocks Zone also for mitochondrial network complexity.
So as drug candidates are proposed, Mirella’s team can repeat this work with cells from an FAer and cells from a non-FAer control, to look at mitochondrial health for each.
Probably the most important project for all of us right now in FA is gene therapy trials. Currently the leading work in those trials already under way in America is on cardiac cells but there’s development work also being done on other organs (teams in Melbourne) and proprioceptor cells (Mirella’s group).
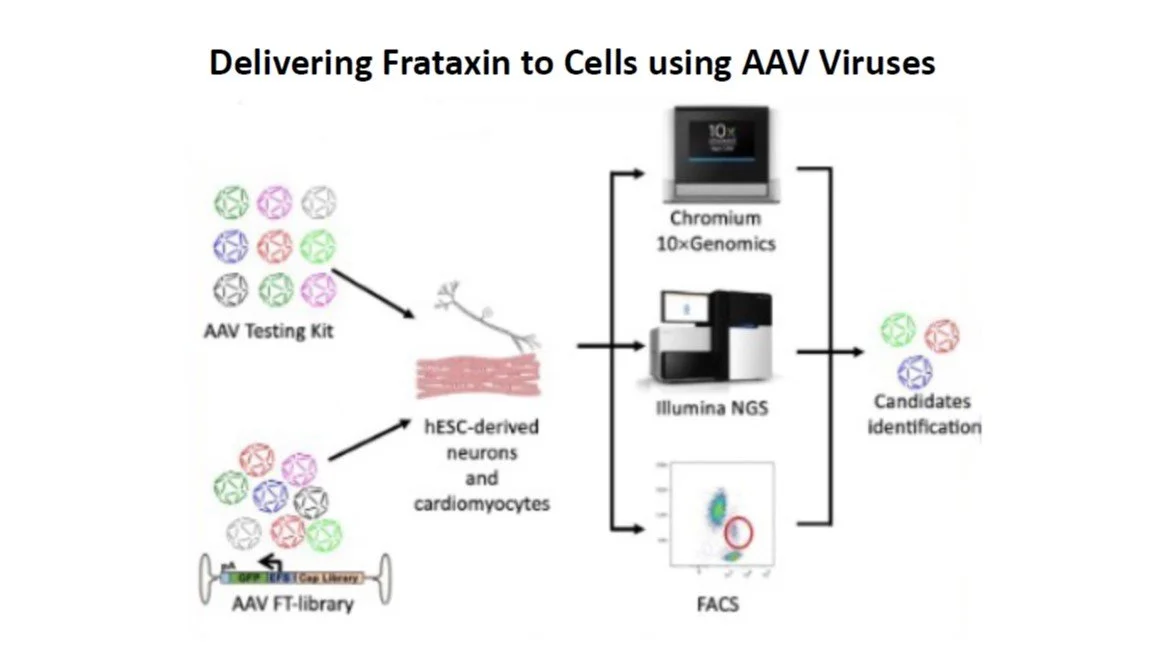
To date there are two approaches in gene therapy. The first involves removing cells from the body (usually bone marrow stem cells), correcting the frataxin gene, then injecting them back into the patient, in the hope that those cells will “teach” other cells in the body that they should be producing frataxin.
The other involves having the frataxin gene “correction” delivered to the cells so it’ll insert itself into the right spot on the DNA strand replacing FA’s trademark GAA repeats and then be present on the DNA every time that cell reproduces itself.
Work is ongoing to establish the optimum way to deliver the correction. Cells have lots of inbuilt protection to avoid anything interfering with their DNA so it must be cleverly disguised. One way being explored is looking at particle types and there’s exploratory work happening with different particle shapes, sizes and even charges, which might resemble the cell types we need to influence so they’d be allowed to get to and deliver the DNA correction where it’s needed.
So far though, the most promising way seems to be to pop the corrected bit of DNA within a deactivated virus. As we all know from recent COVID experience, viruses can get anywhere they want to!
Because FA is such a rare condition the researchers tend to be well connected. In addition to what’s being done overseas, exploratory work on delivering DNA/frataxin correction is being done in Australia currently on the brain, the heart and the liver by other teams as well as on sensory/DRG neurons by Mirella’s group.
This is highly specialised work but these teams also are globally networked to avoid duplication. There are groups of researchers who develop these deactivated viruses, others who take other viruses and bioengineer them; and other groups still who tailor them and the DNA segments they’ll deliver.
The first step is to select the right virus for what it’ll carry and the cells it’ll target. That’s relatively easily checked by having it also deliver a colour dye that can be seen in a photograph.
But all that testing work happens in the lab. Our bodies are complex things, and once they identify the presence of a new virus, antibodies are being generated to kill it and sentries posted so it’ll be recognised if it appears again. The incredibly careful and time-consuming testing for gene therapy trials is often to establish the optimum dose. Because the body’s defences will soon identify and kill a new virus, everything must be delivered first time. There must be enough that it’ll correct enough target cells to make a permanent difference, but not so much that it’ll influence extraneous cells to become cancerous.
This is just the first of a number of such write-ups. Please comment via our FB Group or the email address below if you find them helpful and the work inspiring that these researchers are doing.